As a part of research activities of our laboratory some applicable technologies as well as other
practical results were gained. Here you find the survey of them. For more detailed information you
can contact appropriate people (see hyperlinks).
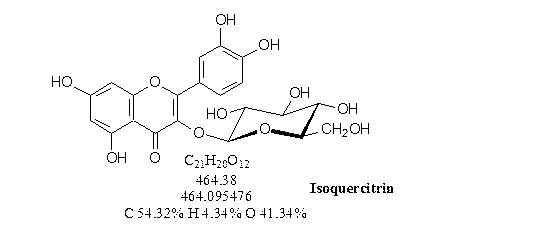
1. Isoquercitrin (Quercetin-3-β-glucopyranoside), Bioquercetin
V. Křen, P. Marhol
Institute of Microbiology, CAS, Prague has developed a proprietary enzymatic technology for a
selective hydrolysis of rutin into isoquercitrin. It is very robust a relatively simple technology
that is biocompatible, without the use of any harmful chemicals and organic solvents.
More details on Isoquercitrin (Quercetin-3-β-glucopyranoside), Bioquercetin
2. Library of glycosidases
More than 70 β-N-acetylhexosaminidases, almost 60 α-galactosidases,
15 α-L-rhamnosidases, and a series of β-galactosidases, α-glucosidases and α-L-fucosidases
from different fungal strains are available as ammonium sulfate precipitates. This preparations
allows structural studies (e.g., crystalization) and prevents impairing of glycosylation patterns
occuring during lyphilization. Preparations, made on the laboratory scale, are fairly stable. The
library of these enzymes, whose synthetic ability was tested, can be used for synthesis of
glycosides from different substrates (combinatorial chemistry) or for selective cleavage (trimming)
of various substarates.
The enzymes are available also on a large scale on request (hundreds – thousands
units).
3. N-Acetylmannosamine containing carbohydrates
Methodology for production of special saccharides containing N-acetylmannosamine covered by
2 patent applications: e.g. p-nitrophenyl α/β-N-acetylmannosaminide,
GlcNAcβ(1–4)ManNAc, GalNAcβ(1–4)ManNAc,
GalNAcβ(1–4)GlcNAcβ(1–4)ManNAc,
GlcNAcβ(1–4)GlcNAcβ(1–4)ManNAc and many others.
Original separation methodology for semipreparatory and preparatory separation of hexosamines and
their derivatives.


 Doc.RNDr. Pavla Bojarová, Ph.D.
Doc.RNDr. Pavla Bojarová, Ph.D.